The Philips Respironics CPAP recall spurred many difficulties nationwide for sleep apnea patients and providers alike, and yet it is not wholly responsible for the ongoing CPAP shortage. There are 3 more reasons PAP devices are hard to get right now, and as a durable medical equipment (DME) supplier, Aeroflow Sleep believes it’s important you understand why. After all, we need your help to make a difference.
A Brief History Of The Philips Respironics CPAP Recall
Although today’s CPAP shortage is not because of the Philips Respironics recall, it is how everything started.
In June 2021, Philips Respironics–a CPAP manufacturer also called “Philips” for short–issued a voluntary recall on 5 PAP devices and 6 mechanical ventilator devices manufactured on or before April 26, 2021, because the polyester-based polyurethane sound abatement foam (or PE-PUR foam) was deteriorating when exposed to high heat; a heat commonly used in CPAP cleaners. Sound abatement foam is used to reduce the noise your CPAP machine makes while it runs, except Philips Respironics’ began to break down. The degraded foam led to black debris entering patients’ humidifiers, tubing, and masks, causing new or worsening symptoms; like headache and cough. In fact, it was found that the chemical emissions made possible by the foam degradation had potential carcinogenic effects.
The following month, the Food and Drug Administration (FDA) identified the incident as a Class 1 Recall, which is declared when “there is a reasonability that the use of or exposure to [this] product will cause serious adverse health consequences or death.” Philips acknowledged the potential health risks and immediately released a recall notification to its affected CPAP users and DME carriers like Aeroflow Sleep, implementing a replacement program where patients could register with the company for replacement devices; including the new Dreamstation 2.
Philips began replacing or repairing DreamStation CPAP machines in September 2021, following on-site inspections by the FDA. Their goal was to have its recall behind them one year later, but the registration process and overall CPAP shortage prevented that goal from being attainable. Moreover, some are still finding out about the recall through blogs like ours, so we’ll help to alleviate your concerns now on Philips’ behalf.
Is my CPAP machine a recalled device?
The well-known DreamStation CPAP, APAP, and BiPAP machines were among the affected devices, regardless of serial number. That series of Philips devices was their best-seller, heavily recommended by healthcare providers until the recall occurred. The full list, made possible by philips.com/src-update, contains:
PAP Devices
- DreamStation CPAP/Auto CPAP/BiPAP (BiLevel PAP)
- DreamStation Go CPAP/APAP
- SystemOne Q Series
- REMStar SE Auto CPAP
- Dorma 400/500 CPAP
Mechanical Ventilator Devices
- E30
- DreamStation ASV/ST/AVAPS
- SystemOne ASV4
- C Series ASV/ST/AVAPS
- Trilogy Evo/100/200 Ventilator
- A Series BiPAP V30 Auto Ventilator
The recall does not include DreamStation 2; however, these CPAP machines are unavailable, because Philips Respironics is solely manufacturing them to provide replacements for recalled devices at this time.
For additional information or questions about an already registered device, we recommend visiting the Philips website to contact them directly. As Aeroflow Sleep receives updates on the Philips Respironics recall, you may also click here to learn about them.
Which PAP devices are approved by the FDA?
Before we resume our discussion about the CPAP shortage as a whole, let’s first take a look at which PAP devices are approved by the FDA and not currently recalled, so you know what to order when the time is right.
Aeroflow Sleep supplies many of ResMed’s PAP devices, including their “For Her” models and their recently-released AirSense 11 (A11) series. The A11 rollout adds two new APAP and CPAP devices to the market (BiPAP devices were not among them,) so you have the opportunity to try some state-of-the-art tech. Plus, the FDA has cleared the “world’s smallest CPAP;” a travel device known as the ResMed AirMini.
ResMed PAP Devices
3B Medical Luna II Auto CPAP
The final CPAP manufacturer backed by the FDA is 3B Medical, and Aeroflow Sleep carries its Luna II Auto CPAP machine. It is equipped with its own data collection system and offers “the most no-cost data capture options in the industry,” allowing you to track your CPAP compliance without compromising on features and benefits. All of this, and the Luna II still has a small physical footprint at only 10.7” x 7.2” x 4.5” even with an integrated, heated humidifier.
Resvent iBreeze Auto CPAP
Thanks to the FDA’s active Emergency Use Authorization, Aeroflow Sleep has also been granted access to an APAP & BiPAP device called the Resvent iBreeze amidst the CPAP shortage and COVID-19 pandemic. It’s perfect for patients who may have struggled with adjusting to the continuous positive airway pressure a standard CPAP emits.
3 More Reasons There’s A CPAP Shortage
Now you know any of the above PAP devices are worth qualifying for supplies through insurance with Aeroflow Sleep, but the matter at hand remains…there’s a CPAP shortage. The possibility of getting one same day is slim at best, and the wait lists can be longer than your arm. There are 3 reasons beyond Philips’ medical device recall that are driving the scarcity:
1. Increased Demand
Half of the nation’s sleep apnea patients were impacted when Philips recalled DreamStation CPAP machines. As such, recalled machines are no longer being made, so that means demand doubled overnight for other CPAP manufacturers. Furthermore, the supply chain is cyclical. CPAP machines need to be replaced every 5 years, so “new” patients continuously become due for fresh supplies.
2. The COVID-19 Pandemic
2021 slowly entered the “post-pandemic” era, but we’re still navigating what that looks like as a healthcare industry one year later. Even society as a whole doesn’t quite know what to do yet, which is why the COVID-19 pandemic continues to upset labor and retention rates across the globe, especially among manufacturers. Fewer people means fewer products; CPAP machines included.
3. Semiconductor Chip Shortages
Finally, there are semiconductor chip shortages too. These critical components are being negligently prioritized for cars and electronics, so manufacturers are literally watching the parts needed to operate your life-saving treatment go to the latest gadget or gizmo instead. Meanwhile, all they can do is keep making the CPAP machines…just at a slower rate.
How You Can Make A Difference
That last reason for why the CPAP shortage is happening lights a fire inside us. We hope it does you as well, because you can make a difference. Aeroflow Sleep is imploring the FDA and other health administrations to take a greater interest in the CPAP shortage, and how you can help us is simple:
- Write to your Congressman
- Write to a technology CEO
- Contact your local media
These are just a few of the ways you can raise awareness and encourage change today. We even have pre-written letters you can print if you’re not sure what to say. Regardless, the most important thing to get across is that a year is long enough. Patients need fresh CPAP supplies for their respiratory care, and Aeroflow Sleep can only do so much.
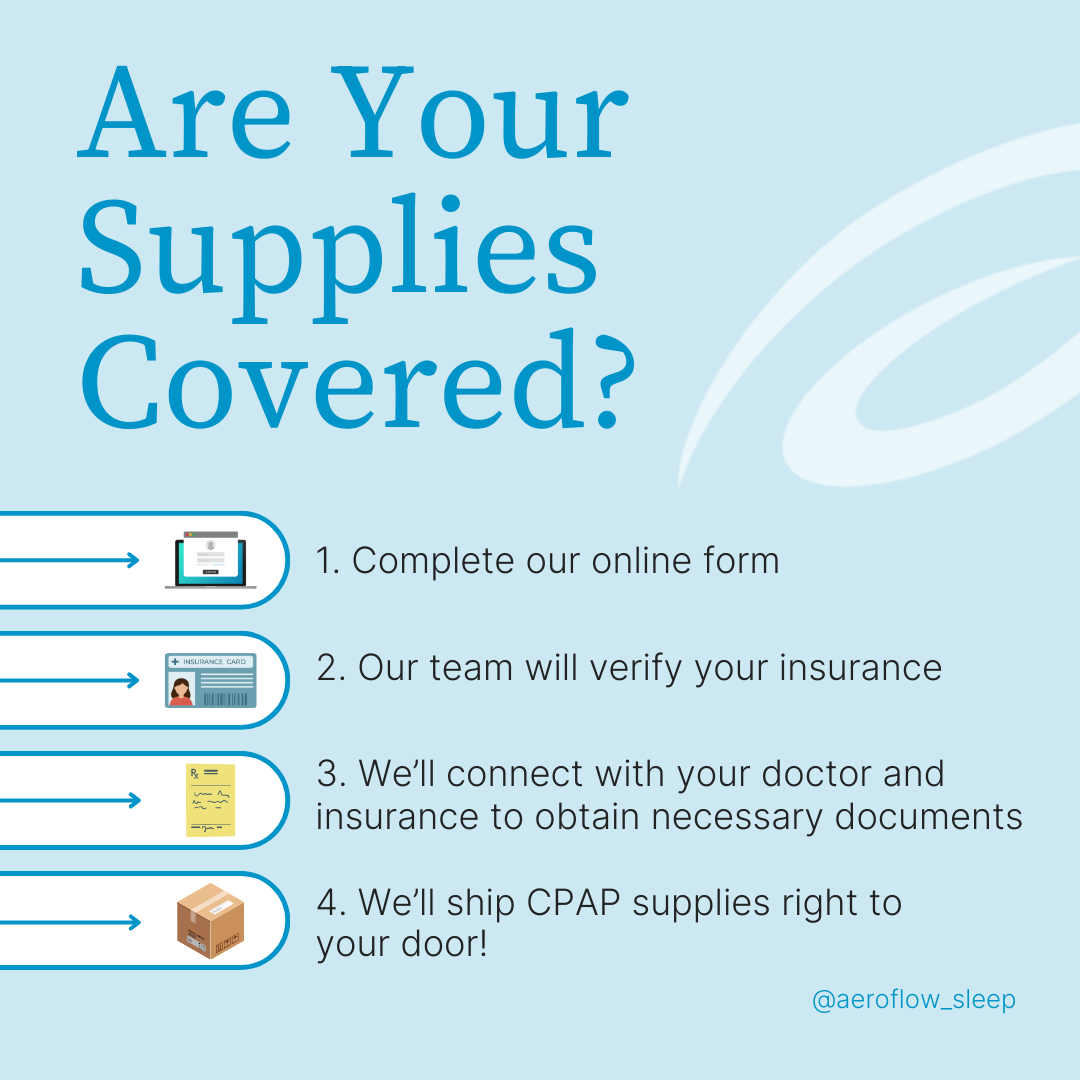
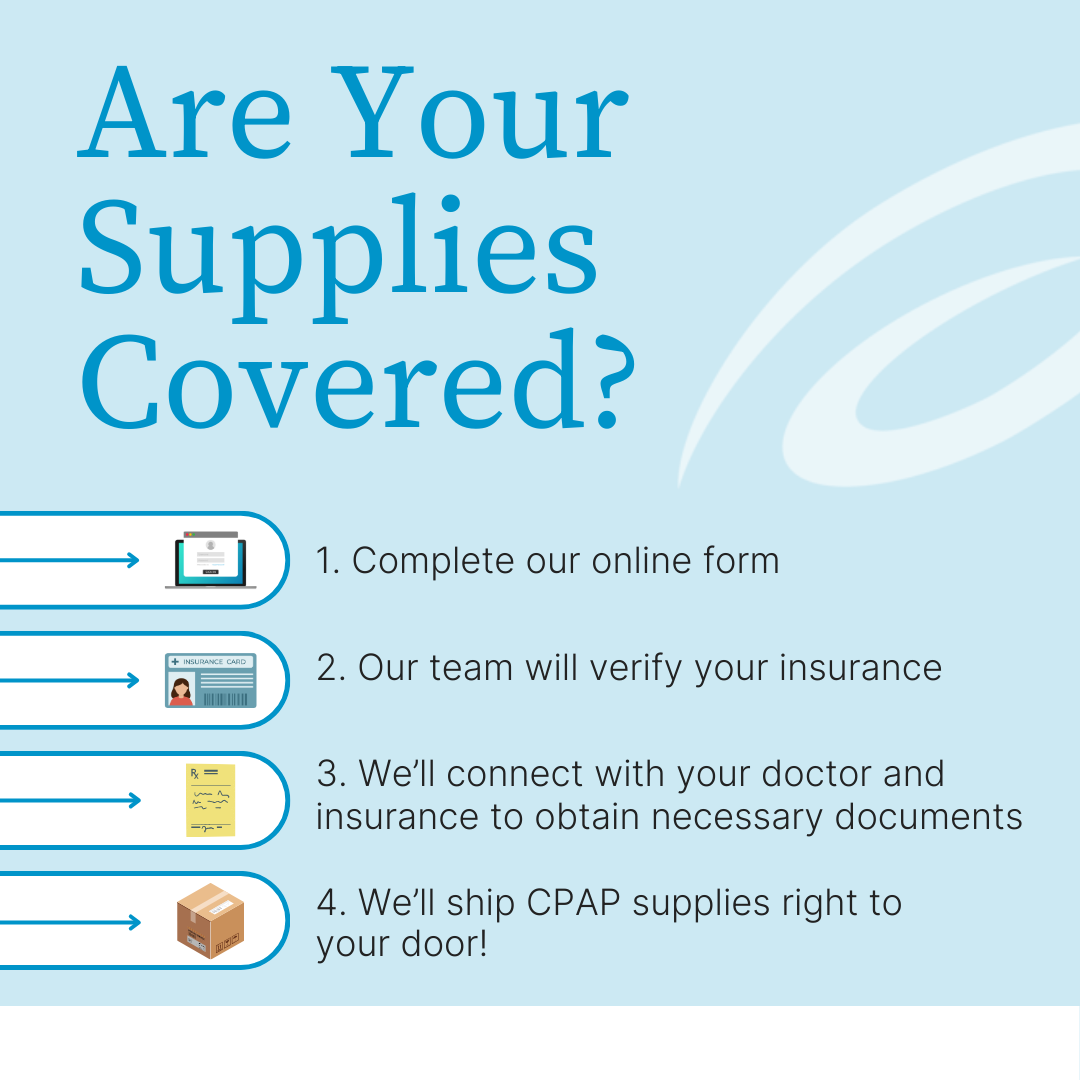
What we can do is help you find a device. If you or someone you love is diagnosed with sleep apnea but still searching for a quick and easy way to get a machine through insurance, please consider us. Aeroflow Sleep will determine if your insurance provider is in our network and work hard to get all of your CPAP supplies covered up to 100%. Just fill out the form found at the link below, and we’ll do our best to keep you happy and healthy on-time.