There’s been a lot of buzz lately about CPAP products the Food & Drug Administration (FDA) has recalled, and while this isn’t necessarily news to anyone in the sleep apnea industry—let alone our patients, why CPAP masks have joined that list may be a question that remains unanswered. That’s why Aeroflow Sleep, alongside Teresa Power DeNike, is here to clear up any misconceptions about magnetic CPAP masks.
What CPAP Supplies Have Been Recalled?
On the off-chance that you’re unfamiliar with the ongoing recall(s,) the following Philips Respironics CPAP supplies and ResMed CPAP masks have been incrementally issued recalls since 2021:
| Philips Respironics PAP Machines | Philips Respironics CPAP Masks | ResMed CPAP Masks |
| DreamStation CPAP/Auto CPAP/BiPAP(BiLevel APAP) | Amara View Full-Face CPAP Mask | AirFit Full-Face CPAP Masks: F20, F20 For Her, F30 & F30i |
| Dreamation Go CPAP/APAP | DreamWisp Nasal CPAP Mask | AirTouch Full-Face CPAP Masks: F20 & F20 For Her |
| SystemOne Q Series | DreamWear Full-Face CPAP Mask | AirFit Nasal CPAP Masks: N10, N10 For Her, N20 & N20 For Her |
| REMStar SE Auto CPAP Machine | Wisp & Wisp Youth Nasal CPAP Mask(s) | AirTouch Nasal CPAP Masks: N20 & N20 For Her |
| Dorma 400/500 CPAP Machine | Therapy Mask 3100 NC / SP | AirFit F20 NV |
You can see the latest additions to this list (and why the media has sparked new conversations about the recall) are not manufactured by Philips Respironics but are ResMed supplies. This came as a shock to some, because Philips had been widely associated with the CPAP recall up until November of last year and rightfully so.
Why Did Philips Respironics Recall CPAP Devices?
Philips Respironics recalled the first column of CPAP devices, because the sound abatement foam that’s used to reduce noise in CPAP machines was breaking down when exposed to high heat. This seems harmless until you consider that many CPAP cleaners and sanitizers use heat as part of their cleaning process. Black debris was found in patients’ masks, tubing, and humidifiers after they put (let’s say) a Dreamstation in their SoClean. The debris was determined to be carcinogenic, and breathing it in also caused many people to experience new or worsening sleep apnea symptoms.
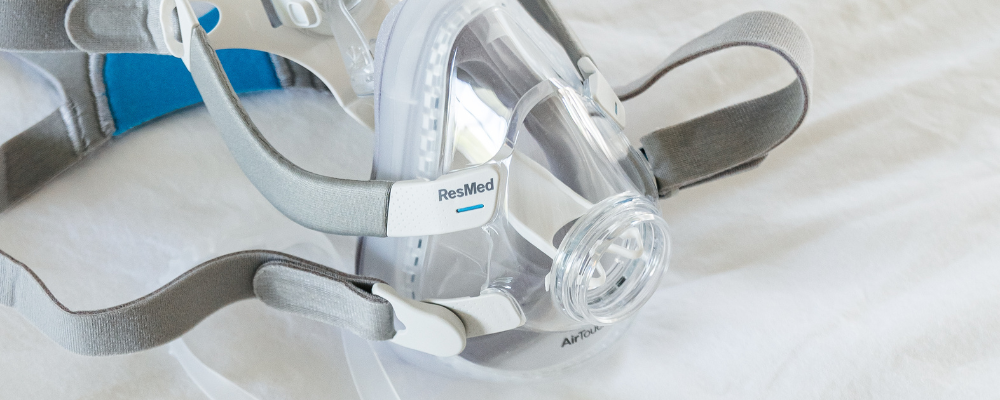
Already under heavy scrutiny, Philips made headlines again but not for its machines. Several of Philips Respironics’ CPAP masks were voluntarily recalled the following year due to safety concerns over magnets found in the headgear. Keep in mind, the magnets were purposely added to the masks, and they were not being recalled due to a malfunction; like the CPAP machines. In fact, the magnets are a fan-favorite feature, because they make the headgear easy to clip on or remove. Unfortunately, the magnets have the potential to interfere with implanted metallic objects; such as pacemakers, stents, cranial plates, etc.
It wasn’t a good look after what happened with the machines, and the FDA continued their investigation into Philips, temporarily overlooking the fact that another manufacturer also develops magnetic CPAP masks. That didn’t last long though, and an urgent safety notice was released by ResMed in November 2023. So, here we are.
Are Magnetic CPAP Masks Hurting You?
The key difference between the machine recall and this is that magnetic CPAP masks are far less likely to hurt you, unless you are a patient with an implanted metallic object. Teresa Power DeNike explains…
“For many of the masks with magnets, this recall [is new.] When we edited last week, the ResMed mask issue was a safety notice... Now, it's ALSO a recall.
"[Futhermore,] keep in mind, ResMed (for example) has issued millions of these masks, and there are less than 10 incidents of serious harm recorded. However, you still want to be mindful of your health and your bed partner’s health; the magnets must be kept at least 6 inches away from anyone who has a metal implant, [so] this might include your bed partner.”
The FDA states that you should replace your magnetic CPAP mask if you (or a person near you; like a bed partner) have…
- Pacemakers
- Implantable cardioverter defibrillators
- Metallic stents (such as aneurysm, coronary, tracheobronchial, and biliary)
- Neurostimulators (such as hypoglossal nerve stimulators)
- Magnetic metallic implants, electrodes, and valves placed in upper limbs, torso, neck, or head
- Cerebral spinal fluid shunts (such as ventriculoperitoneal shunt)
- Aneurysm clips
- Embolic coils
- Intracranial aneurysm intravascular flow disruption devices
- Metallic cranial plates, screws, burr hole covers, and bone substitute devices
- Ocular implants (such as glaucoma implants and retinal implants; intraocular lenses placed during cataract surgery are not impacted)
- Certain contact lenses with metal
- Implants to restore hearing or balance that have an implanted magnet (such as cochlear implants, implanted bone conduction hearing devices, and auditory brainstem implants)
- Magnetic denture attachments
- Implantable ports and pumps (such as insulin pumps)
- Metallic gastrointestinal clips
- Certain metallic joint replacements
- Devices labeled as Magnetic Resonance (MR) Unsafe
- Magnetic metallic implants not labeled for MR or not evaluated for safety in a magnetic field
- Metallic splinters in the eye
- Metallic shrapnel in the body
If you do not meet the above criteria, you’re probably fine to continue using your magnetic CPAP mask. Always consult your doctor or dedicated Aeroflow Sleep Specialist before adjusting your sleep apnea treatment.
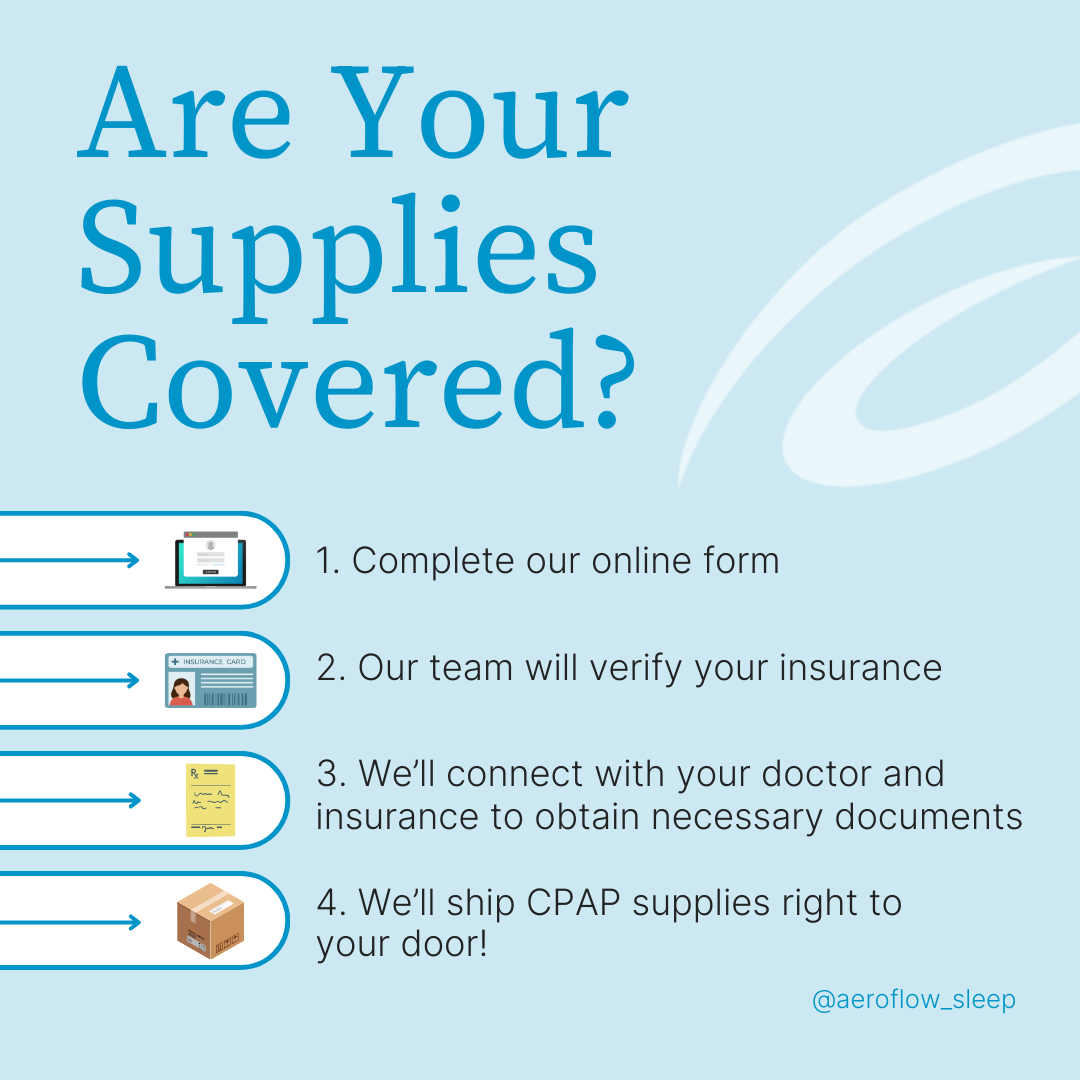
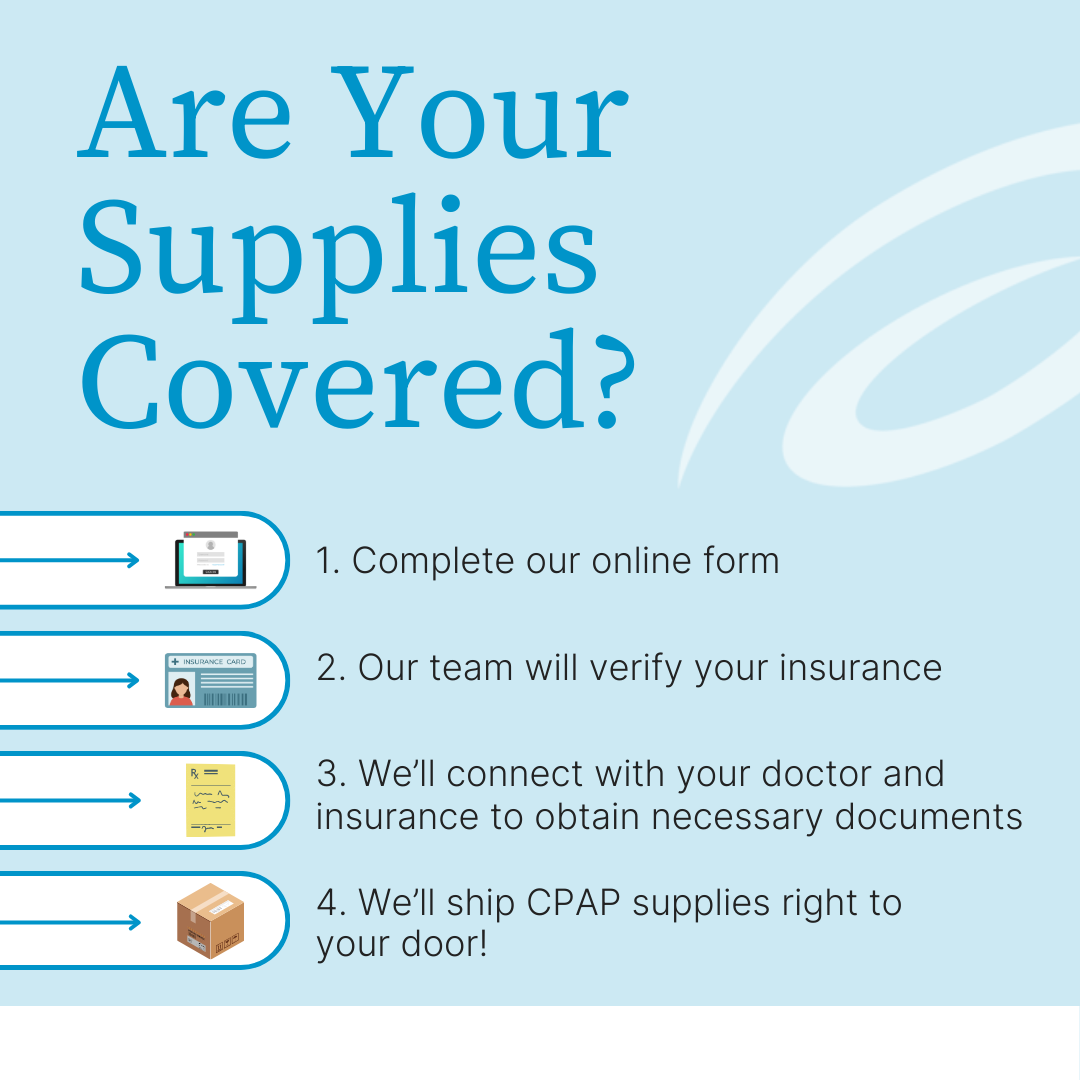
How To Swap Your CPAP Mask
Aeroflow Sleep understands that any recall is scary, especially for patients like you who have endured the last three years of recall whiplash with us. If you’re more comfortable with swapping your CPAP mask, regardless of whether you meet the criteria for a replacement or not, we can help with that.
New CPAP users have 30 days to swap their mask absolutely free of charge when they order from us. Meanwhile, existing Aeroflow Sleep patients can typically replace their headgear (magnetic or otherwise) every 6 months without paying out-of-pocket. Visit our full CPAP replacement guide for more information, or check your eligibility for new CPAP supplies through insurance by clicking below to fill out our online qualifying form. It only takes 5-7 minutes to verify your coverage; private, secondary, Medicare, or Medicaid.
No matter what you choose, we’re ready to help you find the perfect CPAP mask, because your health and wellbeing is our first priority.